The Fundamentals of Inductively Coupled Plasma Mass Spectrometry
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) is an instrumental analytical technique used to measure most of the periodic table of elements. This instrument uses a high-temperature ionization source coupled to a mass spectrometer. It is used in various industries like environmental monitoring, geochemical analysis, metallurgy, pharmaceutical analysis, and clinical research.
Overview of ICP-MS
Learning the fundamentals of ICP-MS is knowing how to use the instrument and obtain its data. Although there are older techniques, ICP-MS is preferred by clinical scientists. ICP-MS is continuously growing with potential for both spectroscopic and non-spectroscopic interference.
Fundamental Compartments of ICP-MS
ICP-MS instruments consist of plasma (ICP), a mass spectrometer (MS), and a detector. A single quadrupole ICP-MS consists of six fundamental compartments: the sample introduction, inductively coupled plasma or the ICP, ion optics, interface, detector, and mass analyzer. Fig. 1 shows the placement of each ICP-MS fundamental compartment.
Samples, usually preferred liquid, are nebulized in the sample introduction method making an aerosol transfer to the plasma. The plasma will then atomize and ionize the sample. After ionization, the sample causes ions to be extracted through the interface territory and into the ion optics.
Meanwhile, the ion optics will direct the ion beam into the quadrupole mass analyzer. The mass analyzer splits ions according to their mass-charge ratio, and these ions are estimated at the detector.
ICP-MS instruments are varied, and the specific design is based on the application type. Sustained by radio frequency, the plasma delivers energy by an induction coil or load coil. After the plasma ionizes the samples, they are transferred to the mass analyzer. So, after being separated, the operator of ICP-MS analyzes the ions detected.
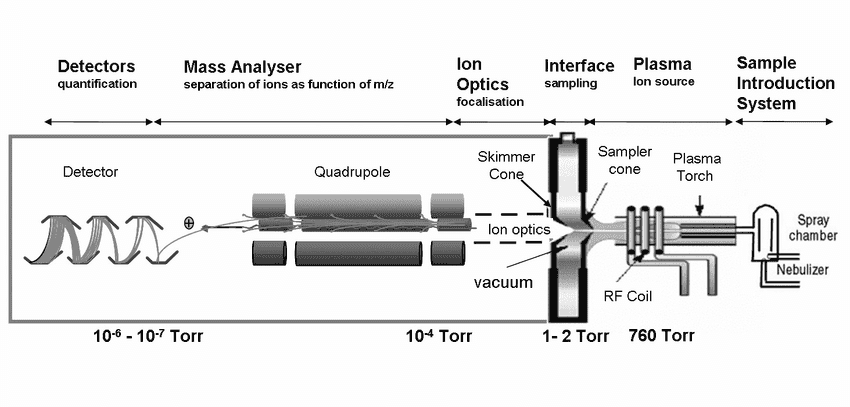
Inductively Coupled Plasma
A plasma is an ionized gas that consists of positively-charged ions and free electrons. The role of the ICP here is to ionize its sample to constituent elements transforming them into ions. It is typically composed of argon gas, and energy is also “coupled” into it using an induction coil to form plasma. It is preferred to use argon gas since the cost of helium is prohibitive.
Interface
The interface is the cones separating the plasma from the mass spectrometer. Generally, the interface is a pair of coaxial nickels where the first cone is called the sample cone, and the second is called the skimmer cone.
The sample cones only allow the central channel of the plasma into the intermediate vacuum region. This material goes through supersonic expansion, pulling ions into the high vacuum region through a skimmer cone.
Ion Optics
Ion optics are located behind the skimmer cone. It is a set of electrostatic lenses. The role of ion optics is to guide the beam toward the mass analyzer. Guiding the beam is done to prevent the photons and other neutral species from reaching the detector.
Mass Analyzer
There are different types of mass analyzers for ICP-MS. After the ions transit through ion optics, they go to the mass analyzer. Mass analyzers can be quadrupole, magnetic sector, and time-of-flight (TOF).
1. Quadrupole mass analyzer
Quadrupole instruments are easy to operate and maintain. Because it is easily operated and maintained, quadrupole instruments are typically used in routine labs. The quadrupole mass analyzer is the most common type for clinical biochemistry applications.
2. Magnetic sector
Magnetic sector instruments are more sensitive than quadrupole instruments. The reason for this is that they use higher ion extraction potentials. Knowing how highly sensitive the magnetic sector is, they are used in more specialized applications or when a higher sensitivity is needed.
Detector
Using detector modes always requires cross-calibration, ensuring optimum linear response. The operation of detectors can both be by pulse and analog. When the signal intensity exceeds a certain threshold, the detectors automatically switch from pulse to analog. Switching detectors allow the linear dynamic range extended by approximately 8–12 orders of magnitude.
ICP-MS Workflow
If used carefully, ICP-MS can be a powerful analytical instrument. Sample preparation and introduction methods must be performed carefully to obtain the best data quality. Checking data acquisition and processing should be done thoroughly. Fig. 2 shows the workflow use of the ICP-MS.
In learning the ICP-MS workflow, you’ll understand the following:
- What sample type can be analyzed by ICP-MS?
- What are the sample preparation and introductions to be considered?
- What factors should be recognized to have an accurate data analysis?
- Understanding the system of ICP-MS
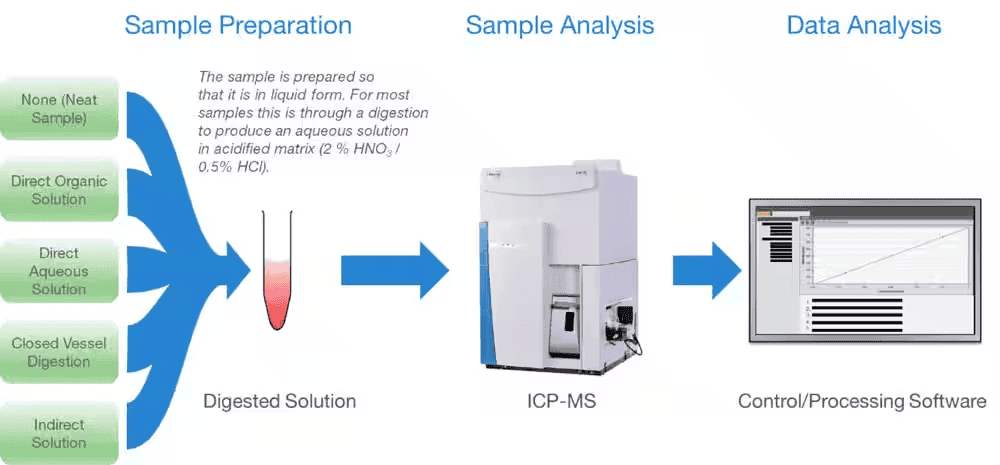
Sample Preparation
Since ICP-MS instruments are used in a wide variety of sample types, they are used in different industries. ICP-MS instruments are designed to analyze liquids that can be dissolved. So before analyzing any sample, it must be well diluted. To prevent nebulizer blockage, the total dissolved content sample recommended for solids is usually <0.2% (2 g/L).
Sample Introduction System
ICP-MS instruments are primarily used to analyze liquids. In analyzing solid samples, electrothermal vaporization or laser ablation is needed. These samples include liquid sample introduction, nebulizer, spray chamber, and hyphenated ICP-MS.
Data Analysis
ICP-MS was first developed 30 years ago, but some laboratories still use older techniques. What ICP-MS analyzes is the number of trace elements in a sample. The instrument must have a reference to a known value to do a quantitative analysis. Generally, the most significant advantage of ICP-MS is its multi-element capability which allows multiple elements to be measured in a single analysis.
Conclusion
ICP-MS is a powerful analytical technique used widely in different industries. It is used to determine trace elements of clinical interest in biological fluids. An ICP-MS instrument consists of plasma (ICP), a mass spectrometer (MS), and a detector. The advantage of this instrument is its ability to measure multiple elements in a single analysis simultaneously.
Providing an extremely low detection limit for nearly all elements it measures is another reason ICP-MS is widely used. ICP-MS can detect many elements below 0.1 part per trillion (ppt). But ICP-MS can also measure elements at concentrations up to 100s or even 1000s of parts per million (ppm). ICP-MS is the only technique that has broad elemental coverage.



Comments are closed.